Eruption!. Create a homemade volcano using baking soda and vinegar to learn about chemical reactions.

Scientific Principles:
- Pressure buildup: Children will observe how pressure builds up within the volcano model before an eruption occurs.
- Gas release: They will learn about the role of gases, such as carbon dioxide, in volcanic eruptions and how they contribute to explosive eruptions.
- Lava flow: By creating a lava-like substance, children will understand the movement and characteristics of lava during volcanic eruptions.
- Chemical Reactions: How an acid and base react to produce carbon dioxide
- Equipment List:
Equipment List:
Baking soda
Vinegar
Dish soap
Food coloring (optional)
Playdough or modeling clay
Small plastic cup or bottle
Tray or basin
Measuring spoons
Funnel (optional)
Safety goggles (optional)
Equipment Required: 2 / 5
Difficulty Rating: 3/5 (supervision and assistance may be required for younger children)


Experiment Description:
The experiment involves creating a ‘volcano’ by placing a container on a tray and filling it with baking soda, a bit of dish soap, and food coloring (for visual effect). Vinegar is then poured into the container, causing a colorful, frothy ‘lava’ to erupt out of the ‘volcano’ and flow down its sides. You will learn about the scientific principles behind volcanic eruptions, such as the role of pressure and the release of gases. Through hands-on exploration, you will discover how magma builds up beneath the Earth’s surface and what happens when it erupts.
Instructions:
Prepare your workspace: Place the tray or basin on a table or any flat surface. This will contain the eruption and make it easier to clean up afterward. Put on safety goggles (if available) to protect your eyes during the experiment.
Build your volcano: Take the playdough or modeling clay and shape it into a volcano cone on the tray. Make it as tall and wide as you want, but leave a hollow space in the center. The hollow space should be large enough to hold the eruption ingredients. You can use your hands or a small tool to mold the clay.
Mix the eruption ingredients: In a small plastic cup or bottle, measure 2 tablespoons of baking soda using a measuring spoon. If you want to add some color to your eruption, you can also add a few drops of food coloring to the cup. Mix the baking soda and food coloring together.
Prepare the vinegar mixture: In a separate cup or bottle, measure 1/2 cup of vinegar using a measuring spoon or simply pouring it in. Add a few drops of dish soap to the vinegar. The dish soap will create a frothy effect in the eruption.
Position the eruption ingredients: Place the cup or bottle with the baking soda mixture into the hollow space of your volcano. Make sure it sits securely so that it won’t tip over when the eruption happens. You can use a funnel to pour the baking soda mixture if it helps.
It’s eruption time! Carefully pour the vinegar mixture into the volcano, directly onto the baking soda. Step back a little to observe the volcanic eruption as it happens. The mixture will react, creating a bubbly and foamy eruption.
Observe and explore: Watch the eruption carefully and notice what happens. Take note of the height and force of the eruption, as well as any colors or textures you observe. Pay attention to how the lava-like substance flows down the sides of the volcano.
Repeat and experiment: After the eruption, you can rebuild your volcano and repeat the experiment as many times as you like. Try varying the amounts of baking soda and vinegar to see if it affects the eruption. You can also experiment with different colors or even add small objects like rocks or toy dinosaurs to see how they get covered in “lava.”
Clean up: Once you are done experimenting, dispose of the erupted mixture by pouring it into a sink or toilet. Rinse the tray, cup, and any utensils used with water to remove any residue. You can save the playdough or modeling clay for future experiments or reshape it into something else.
Simple Explanation: During the experiment, when the vinegar (a type of acid) mixes with the baking soda (a type of base), they react and create a gas called carbon dioxide. This gas needs space to escape, just like when you blow up a balloon. But in this case, the gas builds up pressure inside the volcano model because there’s no way for it to escape. Eventually, the pressure becomes too strong, and the gas bursts out, causing the eruption. It’s like a fizzy soda bottle that gets shaken and then opens with a big splash!
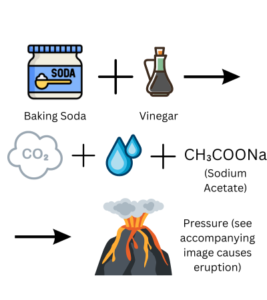
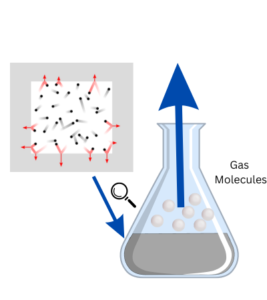
Detailed Explanation: The volcano eruption experiment demonstrates a chemical reaction between an acid (vinegar) and a base (baking soda). When vinegar and baking soda mix, they create a reaction called an acid-base reaction. The vinegar contains acetic acid, and the baking soda has sodium bicarbonate, which is a base. When they come into contact, they react to form carbon dioxide gas, water, and a compound called sodium acetate.
This reaction is an example of a chemical reaction called neutralization. The carbon dioxide gas is produced because the reaction breaks down the baking soda into water, carbon dioxide, and a different compound called sodium acetate. The carbon dioxide gas creates pressure inside the volcano model, just like when a lot of gas builds up inside a closed container.
As more carbon dioxide is produced, the pressure inside the volcano increases. Eventually, the pressure becomes too high, and the gas needs to escape. This is when the eruption occurs. The carbon dioxide gas rapidly escapes, carrying with it some of the liquid mixture from the vinegar and dish soap. The result is a foamy eruption that flows down the sides of the volcano, mimicking the lava flow seen in real volcanic eruptions.
So, the volcano experiment shows how gases, pressure buildup, and chemical reactions are involved in the process of volcanic eruptions.

